FDA publishes requested amendments to PMI?s IQOS application
26th February 2018 - News analysis |
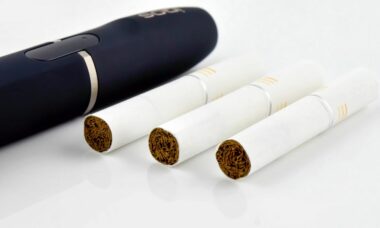
The US FDA has made some public amendments to PMI?s reduced risk applications for IQOS and three types of Marlboro HeatSticks products
The US FDA has made some public amendments to PMI?s reduced risk applications for IQOS and three types of Marlboro HeatSticks products
The American Vaping Association has written to FDA commissioner Scott Gottlieb urging him to approve PMI?s heated tobacco product iQOS for sale in the US
Get 7 days of FREE platform access: Demo our data contents without commitment
"*" indicates required fields
