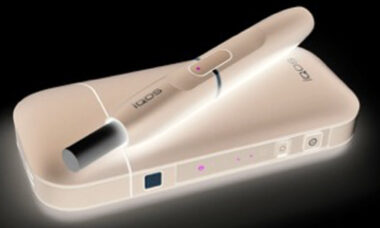
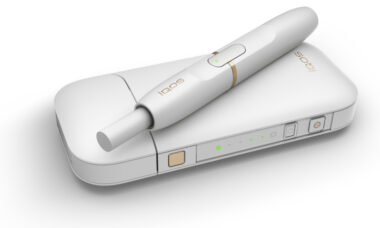
FDA approves its first modified risk tobacco order for eight snus products
23rd October 2019 - News analysis |
The US Food and Drug Administration (FDA) has grated its first ever modified risk orders to eight snus products from Swedish Match, which can now be advertised as less harmful than traditional cigarettes