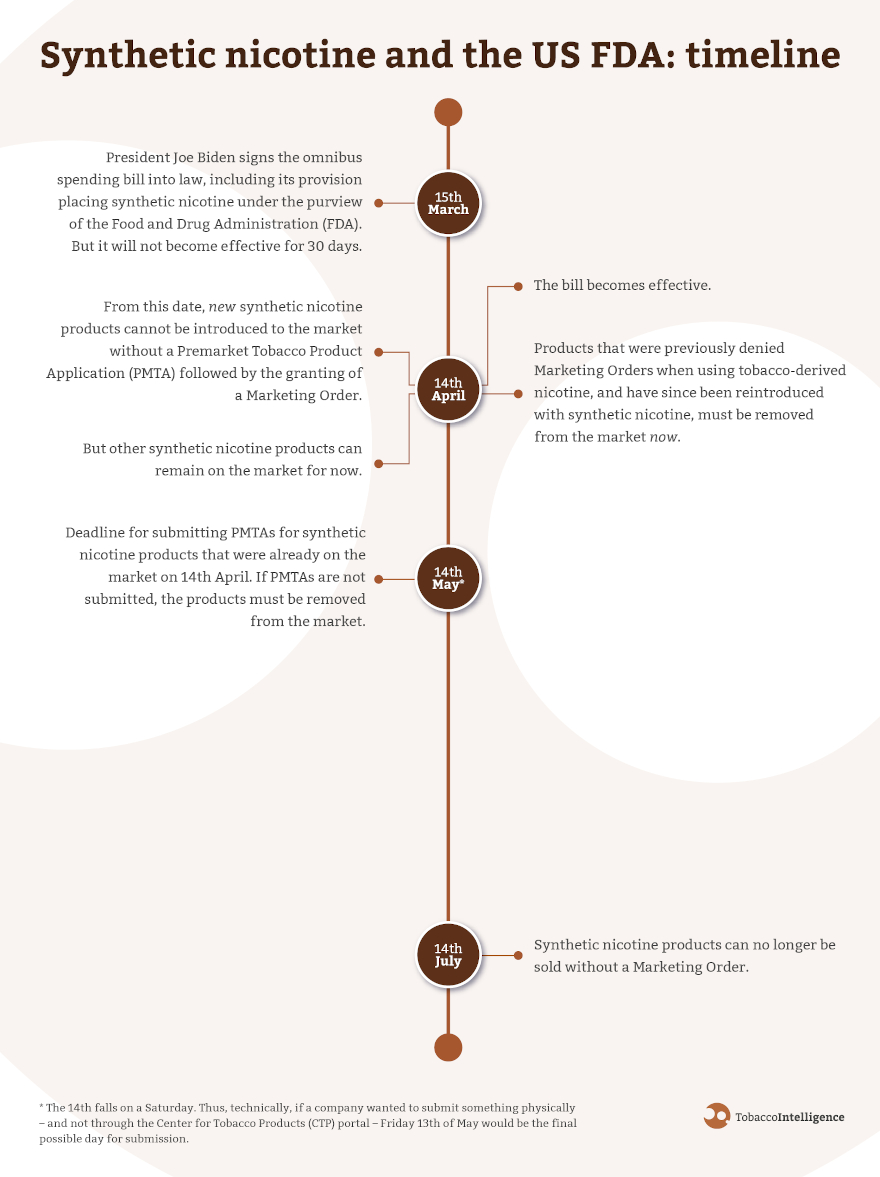
The passage of the $1.5tn omnibus spending bill and its rider giving the US Food and Drug Administration (FDA) authority to regulate synthetic nicotine products in the same way that tobacco products are regulated will irrevocably change the US market.
With a number of major milestones to look out for over the coming months, these are the major points to look out for over the summer as the FDA brings synthetic nicotine into the premarket tobacco product application (PMTA) process.
Experts fear the decision to treat synthetic nicotine like tobacco products could devastate parts of the US vaping industry in particular – an outcome that may have been greeted with a degree of strategic grim delight in the boardrooms of some companies with a tobacco-based application still in play as it may neatly eliminate many potential competitors from the market.
The new legislation will become effective on 14th April, after which new products cannot be placed on the market – while manufacturers will have to submit a PMTA by 14th May for products already on the market to remain on sale.
As the latter date is a Saturday, for any company wanting to submit a PMTA physically – rather than through the Center for Tobacco Products (CTP) portal – Friday 13th May would be the final possible day for submission. All products must have a PMTA in place or be removed from the market as of 13th July.
‘A new and growing threat’
This does not leave companies with much time to put together the necessary information. Due to the short timeframe to prepare PMTAs, they are likely to contain only protocols and timelines for tests and studies, with commitments to submit follow-up amendments with the results so that the FDA can ultimately review robust applications.
The tightening of the rules has been welcomed by some campaigning organisations. Matthew Myers, president of the Campaign for Tobacco-Free Kids, said the organisation supported the measure and hoped it would lead to a crackdown on flavours that are “attracting and addicting kids”.
“This agreement is an important victory for our nation’s kids and public health,” Myers said. “Synthetic nicotine poses a new and growing threat to the health of our nation’s kids.”
Beyond the need to submit a PMTA, manufacturers of synthetic nicotine products will be subject to all the regulations for tobacco products. This likely includes all other Tobacco Control Act requirements, including tobacco product establishment registration and product listing, ingredient listing, label compliance, and health document submissions.
– Antonia Di Lorenzo TobaccoIntelligence staff
Graphic: Carl Gamble







