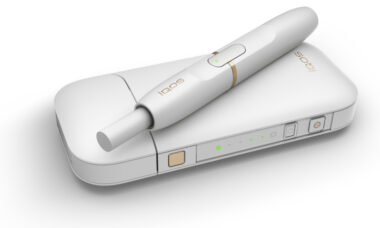
US tobacco regulator responds to critical report with promise of five-year plan
28th February 2023 - News analysis |
The US Food and Drug Administration (FDA) has announced its plans to address the recommendations put forward in a damning independent review of its Center for Tobacco Products (CTP)