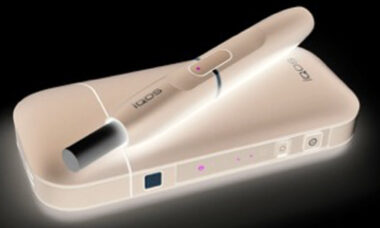
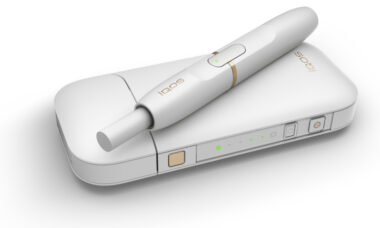
Altria reports solid oral tobacco segment performance despite lower revenues
2nd August 2023 - News analysis |
Altria reported a solid performance in the first half of 2023 despite lower net revenues, partially offset by higher revenue in the oral tobacco segment